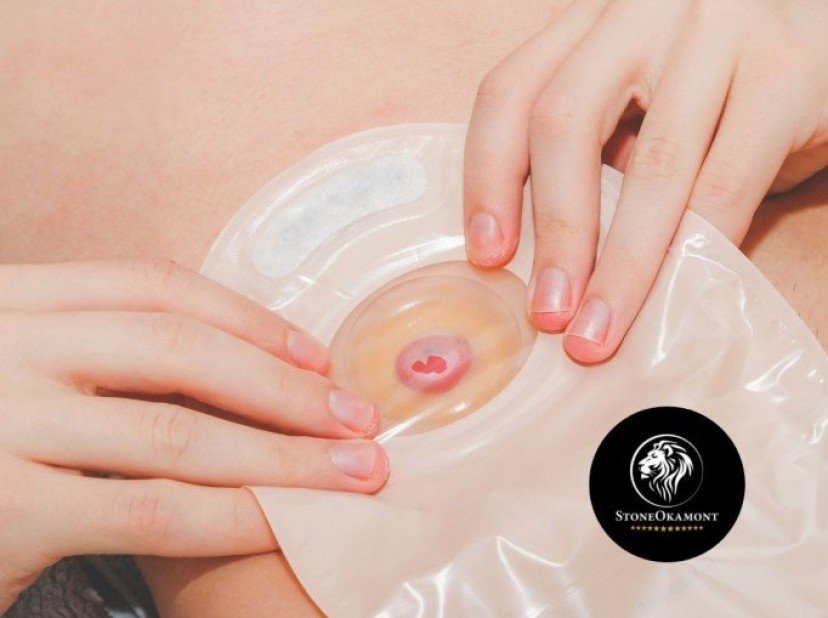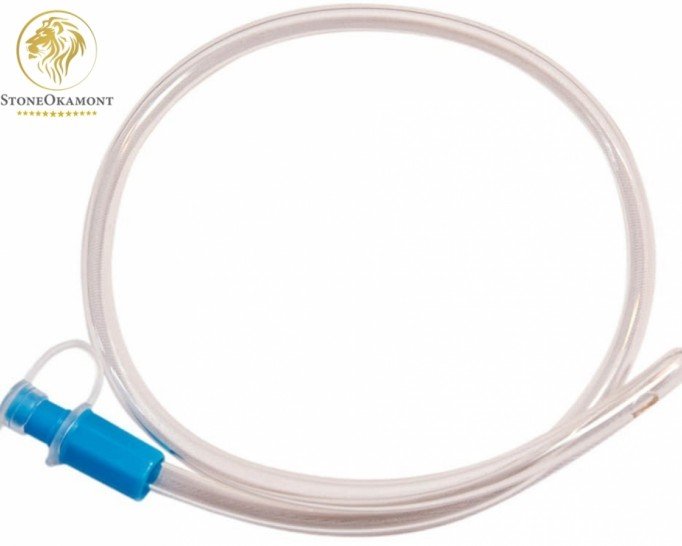
Urethral probe is a sterile device inserted through the urethra with the intention of draining fluids from the bladder or urethra, it can also be used to collect material for examination or to direct medication, hydration or contrast.
The probe is made of non-toxic flexible PVC, sterile and single-use product. (disposable)
Anvisa (National Health Surveillance Agency) classifies the urethral probe as a correlate (Health products) with risk class II - Medium risk, and it is not necessary to obtain the Good Manufacturing Practices to register a urethral probe in Brazil. GMP is mandatory for companies that operate with products classified as: risk III - High risk and risk IV - Maximum risk. Good Manufacturing Practices are a set of norms and rules that establish standards related to the processes that involve the product.
Anvisa (similar to the FDA) is the Brazilian agency responsible for regulating products: correlates, medicines, cosmetics, sanitizing products and others. Every product entering Brazil needs to be regularized before its sale.
Do you know what it takes to register urethral probe in Brazil? In this content we talk about each regulatory step necessary to register urethral probe in Brazil.
Request a budget
What is needed to register urethral probe in Brazil?
To register a urethral probe in Brazil, it is necessary to present safety and efficacy tests to ensure that the product fulfills the purpose for which it was produced without causing any harm to the final consumer. In addition, it is necessary to present information about the product, such as: composition material, use, disposal form, among others.
Before registering products in Brazil, the company must be properly regularized with Anvisa.
Below are two regularization options for registering urethral probe in Brazil.
Option 1: Company regularization
The first step in regularizing the company to register a urethral probe in Brazil is to establish a physical location following the requirements of Visa and Anvisa.
The second step is to obtain the Operating License. This is requested from Visa (Local Health Surveillance), in this process the company must adapt its physical structure according to the RDC (Resolution of the Collegiate Board) corresponding to the activity performed for subsequent inspection by a Visa inspection agent.
After the municipal or state regularization (Operating License), the process moves to the federal level and must be protocoled in Brasília. The Operating Permits, unlike the first process, is analyzed by Anvisa. At that moment, there are document analysis, collection of fees, verification of petitions and others.
Option 2: Holder service
Choosing Holder Service your company has much more freedom in bringing its products! This option allows to bring the products through Stone Okamont, using the -BRB Holder without the need to establish a physical company.
Contact Stone Okamont!
In order to register a urethral probe in Brazil without complications, the assistance of a competent consultancy is essential. Stone Okamont is the right consultancy for your business! Count on us to register urethral probe in Brazil and avoid unnecessary expenditure of time and money. With Stone Okamont, processes are quick and easy.
Don't waste any more time! Fill out the form below, talk to one of our professionals and ask all your questions about how to register urethral probe in Brazil.